The amino guarantee

60 Day
Money back guarantee
We are driven by the simple idea that great science yields great products. All of our products are clinically tested for safety and effectiveness, so we know you'll love them.
We guarantee it.
Real people, real results
I look forward to working out because I know I get to enjoy my Perform shake. I have way more stamina and strength and don’t feel drained after my workout.”





Danny US
- Health Concerns: Endurance, focus, strength
- Product(s) Purchased: Perform
- Usage: 2 scoops Perform
Heal helped me recover so much faster from my hip replacement surgery. I’m feeling stronger every day and able to walk without pain for the first time in years.





Mary US
- Health Concerns: Recovery after surgery
- Product(s) Purchased: Heal
- Usage: 3 scoops Heal
Life has helped me regain my energy and my muscles. I’m turning 55 this year, and I’ve never looked or felt better.





Allan US
- Health Concerns: Aging, muscle loss
- Product(s) Purchased: Life
- Usage: 2 scoops Life every morning with breakfast
Our science is unmatched
This team is on the cutting edge of modern medical nutrition, and they lead the world in developing great products that improve lives.
Dr. Simon Chambers, United States

30+ Years of clinical research
500+ Peer-reviewed publications
70,000+ Times cited by other researchers
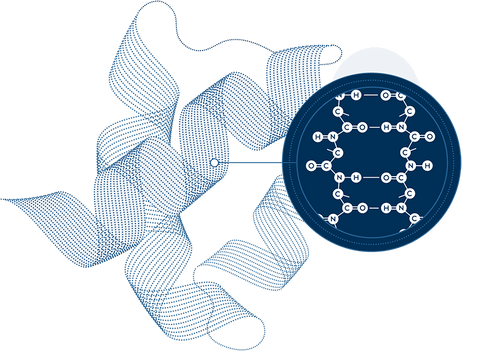
Our secret is in the science
Small but
mighty
Amino Acids are our greatest asset. As the building blocks of protein and human life, just 21 amino acids create thousands of proteins – and enable nearly all biological functioning.
Emphasis on the essentials
Our bodies can only make 12 of the 21 amino acids, which means food must provide the other 9. These 9 are called essential amino acids (EAAs), because they’re vital to our existence.
Precise ratios, powerful results
Because EAAs are most powerful when consumed in exact ratios, our research is grounded in the development of precise EAA profiles. Once perfected, our patented profiles deliver optimized, targeted, and truly unparalleled health results.
Proven and Unique
All products are patented or patent pending, with proven superiority through clinical trials.
Research Focused
Our work is based on 40 years of research, over 100 clinical trials, and over 500 peer-reviewed papers.
Safe and Effective
Clinically tested to ensure maximum absorption with no harmful side effects.
Clinically proven products
Amino Company products are built on amino acid technology first funded by NASA and further refined through rigorous research and independent clinical trials.
We’ve determined the precise blends of amino acids to help you become stronger, heal faster, reduce age-related declines, and improve your overall metabolic health.
Free e-book
Sign up for our newsletter and let us know what you’re interested in, and you’ll also receive a free E-Book.
30 years of research... and still going.

60 Day
Money back guarantee
The amino guarantee
Give us a try today.
If, for any reason, you don’t like us or our products, simply contact our support team within 60 days and we’ll happily refund you 100% of your payment.
It's our way of making sure you're completely happy with your purchase.


 833-264-6620
833-264-6620